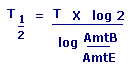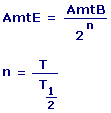| Half-Life Calculator |
|
|
|
|
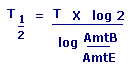 |
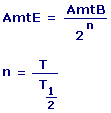 |
T1/2 = Half Life
T = Elapsed Time
AmtB = Beginning Amount
AmtE = Ending Amount |
Click on Calculate Time or Calculate Half-time or Calculate Beginning Amount or Calculate Ending Amount you wish to calculate.
Enter value and click on calculate. Result will be displayed.
|
|
The Half Life Time of a quantity whose value decreases with time is the interval required for the
quantity to decay to half of its initial value.
The term Half Life Time was coined in 1907.
|
The Half Life Time is the amount of time it takes for half of the atoms in a sample to decay.
Half Life is a characteristic of each radioactive isotope.
Depending on the isotope, its Half Life may range from a few fractions of a second to several billion years.
The Half Life of Uranium-235 is 713,000,000 years. The Half Life of Uranium-238 is 4,500,000,000 years.
There is even a radioactive isotope of carbon, carbon-14.
Normal carbon is carbon-12. C-14 has two extra neutrons and a half-life of 5730 years.
Scientists use C-14 in a process called carbon dating.
Carbon dating is when scientists try to measure the age of very old substances.
The Half Life is independent of the physical state (solid, liquid, gas) temperature, pressure,
the chemical compound in which the nucleus finds itself, and essentially any other outside influence.
It is independent of the chemistry of the atomic surface, and independent of the ordinary physical factors of the outside world.
The only thing which can alter the Half Life is direct nuclear interaction with a particle from outside, e.g.,
a high energy collision in an accelerator.
|